Snail Fever Vaccine Reaches First Human Clinical Trial
Long journey to prevent major tropical disease is worth the wait
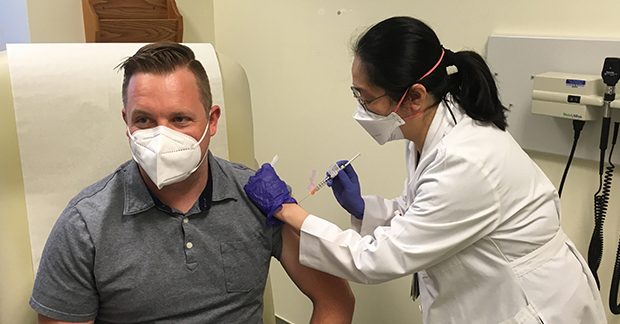
Participant in clinical trial receives world’s first-ever injection of SchistoShield
After decades of hard work, a preventative vaccine for major tropical disease schistosomiasis has reached a Phase 1 clinical trial, providing a dose for the first healthy human volunteer and paving the way into a better future for the 74 countries and nearly one billion people currently threatened by this disease.
Afzal A. Siddiqui, Ph.D., Grover E. Murray Distinguished Professor with Texas Tech University Health Sciences Center (TTUHSC) School of Medicine, served as lead researcher and invented the schistosomiasis vaccine, called SchistoShield®. This vaccine was brought about by PAI Life Sciences, a biotechnology company specializing in translational research for neglected tropical diseases.
According to Siddiqui, it is the result of many years of hard work and research.

Afzal A. Siddiqui, Ph.D.
“It has been 30 years of a bumpy ride at a snail’s pace to bring SchistoShield® vaccine for snail fever [schistosomiasis] to first in human clinical trials,” said Siddiqui.
Schistosomiasis (or ‘snail fever’) is a disease caused by parasitic flatworms predominantly found in sub-Saharan Africa, Middle East, Southeast Asia and the Caribbean. These worms reach humans through the skin when it comes into contact with contaminated water. Symptoms of schistosomiasis include abdominal pain, diarrhea and blood in stools and/or urine. With the potential to cause long-term liver damage, kidney failure and death, a clinically proven vaccine could change the course of the future for millions.
“Development of a vaccine that is effective against geographically distinct forms of schistosomiasis is essential to make a meaningful impact in global reduction of disease burden,” said Siddiqui. “A low-cost, effective vaccine for schistosomiasis would greatly aid in the fight against this debilitating disease. The significance of successful clinical trials is clear in that an approved, effective, deployable vaccine for this disease would impact as many as one billion people in 74 countries throughout the world.”
In May of this year, TTUHSC achieved Carnegie Classification® status for Special Focus Four-Year Research Institutions.
The clinical trial will evaluate the safety and the immunogenicity of PAI Life Sciences’s SchistoShield® vaccine in 45 healthy adults between the ages of 18 and 55, and is sponsored by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Related Stories
TTUHSC Cancer Researcher Honored by National Academy of Inventors
C. Patrick Reynolds, M.D., Ph.D., director of the School of Medicine Pediatric Cancer Research Center at TTUHSC, has dedicated his life as a researcher to developing treatments for childhood cancers.
TTUHSC’s Hudson Set to Serve as President for Society of Clinical Research Associates
The Society of Clinical Research Associates (SOCRA) has elected Texas Tech University Health Sciences Center’s (TTUHSC) Catherine Hudson, Dr.P.H., as its president for 2025-2026.
Clinical Research Institute a Source of Pride for Retiring Griswold
Upon his retirement, John Griswold, M.D., reflects on the Clinical Research Institute and what it has achieved.
Recent Stories
The John Wayne Cancer Foundation Surgical Oncology Fellowship Program at Texas Tech University Health Sciences Center Announced
TTUHSC is collaborating with the John Wayne Cancer Foundation and has established the Big Cure Endowment, which supports the university’s efforts to reduce cancer incidence and increase survivability of people in rural and underserved areas.
TTUHSC Receives $1 Million Gift from Amarillo National Bank to Expand and Enhance Pediatric Care in the Panhandle
TTUHSC School of Medicine leaders accepted a $1 million philanthropic gift from Amarillo National Bank on Tuesday (Feb. 10), marking a transformational investment in pediatric care for the Texas Panhandle.
Texas Tech University Health Sciences Center Permian Basin Announces Pediatric Residency Program Gift
TTUHSC Permian Basin, along with the Permian Strategic Partnership and the Scharbauer Foundation, Feb. 5 announced a gift that will fund a new pediatric residency.
